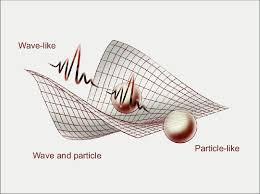
Wave-Particle Duality
In 1924, De Broglie proposed that all matter exhibits wave-like behavior. His hypothesis led to the development of quantum mechanics and was later confirmed by electron diffraction experiments.
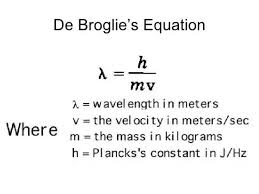
De Broglie Wavelength
De Broglie derived the equation λ = h/p, where λ is the wavelength, h is Planck’s constant, and p is the momentum of the particle. This equation describes the wave nature of particles.
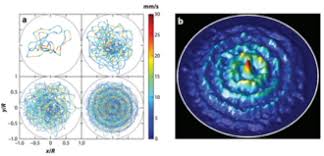
Pilot Wave Theory
He introduced an alternative interpretation of quantum mechanics, suggesting that particles are guided by an underlying wave function, a concept explored later in Bohmian mechanics.
DID YOU KNOW?
• Louis De Broglie was awarded the 1929 Nobel Prize in Physics for his discovery of wave-particle duality.
• His work influenced Schrödinger in formulating the wave equation.
